Chlorinated and fluorinated — including Group 7 elements — solvents have enjoyed decades of popularity as robust and efficient means of cleaning parts as a first critical step in many metal finishing processes.
 Stephen Rudy CEFThese organic halide solvents are classified as:
Stephen Rudy CEFThese organic halide solvents are classified as:
- Hydrochlorofluorocarbons (HCFCs)
- Hydrofluorocarbons (HFCs)
- N-propyl bromides (nPB)
- Chlorofluorocarbons (CFCs)
- Perfluorocarbons (PFCs)
Their specific applications include cleaning and degreasing of electronics, such as printed circuit boards and semiconductors, automotive, carbon removal, intricate and sophisticated parts cleaning, and as drying agents.
Solvent types among these have been: trichloroethylene, 1,1,1-trichloroethane, perchloroethylene, methylene chloride, and n-propyl bromide. These solvents are still in use for their intended purposes, affording reliable sources for cleaning parts in a wide array of industrial manufacturing and finishing applications. These solvents became targeted for replacement due to toxicity, worker related and environmental hazards, and stricter requirements for their handling and use in today’s work environment.
Continuing their use has entailed expensive, compliant containment, filtration, and exhaust mandates. Manufacturers of these products have encountered higher production costs. These expenses along with special taxes are passed on to the end user.
Main image courtesy of Turbex, https://www.turbex.co.uk
Steady Decline in Upper Atmospheric Ozone Layer
Going back to the 1970s to 1980s, applied research and space satellite data analysis detected a steady decline in the upper atmospheric ozone layer. This layer, protecting the earth’s surface from harmful ultraviolet radiation, would steadily lose its shielding ability. This would detrimentally affect populations on Earth to potential health problems when exposed to prolonged sunlight. Ongoing scientific work and study confirmed that particular solvents, containing chlorinated and fluorinated compounds, were highly suspect. Evaporation and release of these solvent containing compounds into the stratosphere, resulted in their chemically attacking the ozone layer. This continued chemical attack led to a gradual, alarming degradation of the ozone layer.
Two classes of these compounds were identified: chlorinated fluorocarbons (CFC) and chlorinated hydrocarbons (CHC). Two major sources of CFCs were in heavy use aerosol propellants and refrigeration gases. CHCs in demanding applications included dry cleaning of clothing, separation of materials and solvency to prepare various consumer and commercial products, hard surface and precision cleaning, and in food preparation.
Overall depletion of the earth ozone layer became alarmingly critical to several organizations. These included educational interests, environmentalists, industries, governments and many other concerned groups. The common theme to action was to be a good steward of our world, thereby ensuring the safety of future generations. Some of the key actions developed throughout the years are familiar to many of us. These include:
- 1990 Montreal Accord
- S. Clean Air Act Amendments
- S. Clean Water Act
- VOC and HAP regulations
- Hazard communications
- RCRA
- CERCLA
- Superfund
- Strict regulations
- Compliance mandates
Additional Studies on Health And Safety
Actions and triggers, such as those described, led to additional studies on the health and safety associated with these targeted solvents. Toxicological data was quantitatively determined. Potential acute health effects were established. Of these, perhaps, the most critical was OSHA’s classification of some of these solvents as carcinogenic. Actual exposure limits (in ppm and mg/m3) were also determined, as strict requirements in the affected workplace.
This resulted in new, more stringent engineering controls for operating equipment, such as vapor degreasers. It is a relatively simple concept to control airborne concentrations of vapors below their respective threshold limits. However, to achieve this at very low ppm levels, as mandated, does result in quite expensive equipment control devices. Ecological considerations include byproducts of biodegradation. In this respect, long term degradation products are more likely. In fact, the degradation products may be more toxic than the parent compound.
ome of these solvents, such as trichloroethylene, 1,1,1-trichloroethane, methylene chloride, perchloroethane (perc, trichloroethylene) are Class 6.1 (Poisonous Material). Perhaps most clear in caution is California Proposition 65, which states these CHCs have been found to cause cancer.
For those installations still using CHC cleaning agents, the required compliant equipment must be used, to meet the critical airborne exposure limits. For those installations that eliminated targeted CHCs, almost two-thirds focused on eliminating solvents. Almost the same fraction considered replacing these solvents with aqueous cleaners.
Replacing chlorinated and fluorinated solvents in general bulk and precision cleaning can be challenging to achieve. These cold or warm solvents typically provide cleaned, dry parts in one to three minutes. These solvents also remove metal chips, fragments and shavings from small parts, such as screws and rivets. Solvents are regenerated and purified by distillation.
Aqueous Cleaning
Let’s consider the application of aqueous, or water based cleaning, as an alternative to the described solvents. Our industry has utilized and thrived with aqueous cleaning technology for many decades. It has always been with us, upgraded, improved, to satisfactorily clean parts, as a first step in a multitude of finishing processes.
Operations such as: plating, anodizing, phosphating, black oxide, chromate, painting, passivation, electropolishing and more, attest to the versatility of aqueous cleaning. In these line cycles, it is almost universal to employ a soak cleaner.
Soak Cleaning Background
Stable concentrates are available in powder and liquid forms. These commercially blended products are formulated based on types of metals being processed (ferrous and non-ferrous), desired cleaning mechanism (displacement or emulsification), types of soils to be removed, temperature range, analysis and control, and disposal considerations.
Based on application, blends may consist of alkalinity builders, caustics, water hardness conditioners, surfactants, wetting agents, deflocculants, inhibitors, and metal reducing agents. Operating baths can be either high or low foaming, accommodating mechanical agitation considerations. pH sensitive metals, such as aluminum, brass and zinc, can be efficiently soak cleaned (no etching) in mildly alkaline cleaners versus the higher pH, or caustic containing cleaners prevalent for steel.
Acidic cleaners are also used to remove organic soils as well as oxide scales and rusts off base metals with the added benefit of chemical surface polishing. In some applications mild pH soak cleaners (pH 6-9) may apply to both ferrous and non ferrous metals in a single step.
Equipment Requirements
- Suitable tank construction, such as stainless steel, plain steel and plastics. The materials of construction should tolerate anticipated heating requirements and be tolerant to the specific cleaner chemistry.
- Heating is very important. Most aqueous systems require a temperature range of 140°F -180°F (60°C -82°C), for optimized cleaning cycles that may range from 3 to 10 minutes. Steam coils, electric immersion or gas fired sources for heat are typical. A rule of thumb is double cleaning efficiency with every ten degree rise in temperature.
- Agitation can be very important and critical to successful soak cleaning. Solution movement facilitates soil removal and promotes uniform solution temperature.
- This is a very important complement to maintain optimized cleaning. Solids, oils and grease are continually removed. This also extends the cleaner bath service life, thereby minimizing dumps. This reduces the work load in waste treatment, and keeps desired line production operating longer between maintenance down time.
Soak Cleaning Action
Oily soils, grease, fine solids, polishing and buffing compounds, etc... are typically removed by either emulsification or displacement. Unique chemical formulations, balancing alkalinity with specific surfactants and wetting agents, can provide either type of cleaning action. Emulsification suspends and holds the soils in solution. An effective soak cleaner, properly maintained, should hold a range of 10 15% of these soils, to bath exhaustion.
In displacement cleaning, the soils are released by a different cleaning mechanism. Unlike emulsification, in displacement, the soils are not held in a chemical “cage,” but allowed to settle out physically, separating from the aqueous phase. Most oily organic soils are less dense than water, and will float to the surface of the cleaner bath.
Many cleaner tanks incorporate an overflow weir into a small side tank for recirculating the solution. This is an ideal place to install and operate a belt skimmer or disk, to remove the separated and collected oily soils. In fact, any cleaner can benefit from oil removal. During operation there can be at least a 20 degree cooling of the solution in the overflow, resulting in more oils separating. Even emulsifying cleaners will release some oils upon cooling.
Small steel stamped parts are usually heavily oiled. Displacement cleaning in these types of barrel applications, especially in tandem with oil removing equipment are all around beneficial.
Soak Cleaners Analysis and Control
Alkalinity titration, to an indicator, or pH endpoint has been the most accepted, readily available, and quickest method. It is equally applied for liquid and powdered type cleaners. Based on analysis to neutralize alkalinity, a quantitative amount of product concentrate is added to adjust and maintain desired operating concentration. By this method of analysis, the wetting agents, surfactants and other non titratable components in the cleaner are not determined. However, by adding the cleaner as a whole product, this usually provides a sustainable, working balance of all components for effective cleaning. The alkalinity titration also applies to multi-component cleaner systems, such as separate alkalinity and detergency additives.
Titration to a determined endpoint provides a quantitative addition of the alkali additive. By the operating ratio, the detergency additive is calculated, and added to maintain the desired operating ratio of both in the bath. A benefit of using liquid cleaners is the ability to incorporate continuous analysis by measuring the cleaner bath conductivity. This, in turn, can automatically dispense cleaner concentrate to maintain desired operating conductivity, minimizing rejects due to poor surface cleaning.
Other supporting analyses of the cleaner can be incorporated to monitor age, soil loadings, and if effective service life has been achieved. Usually a ready-to-dump cleaner can be tested for specific levels of contaminants. These values can be used as a guide to monitor service life of a newly made-up cleaner. Examples include metal contaminants (e.g., chromium, copper, iron and zinc), extent of soil emulsification, solution density, and effect of water break test on special cleaned coupons.
Soak Cleaner Waste Treatment
Spent solutions are effectively treated by adjusting pH, releasing emulsified oily soils, precipitating metals and filtration. Treated solution is discharged per local POTW or municipal waste water authority regulations. Depending on plant processes, treated water may be recycled for use in non critical applications.
Complete waste treatment cost of a spent aqueous cleaner may be one quarter or less when compared to proper shipment and incineration of spent halogenated solvent cleaners.
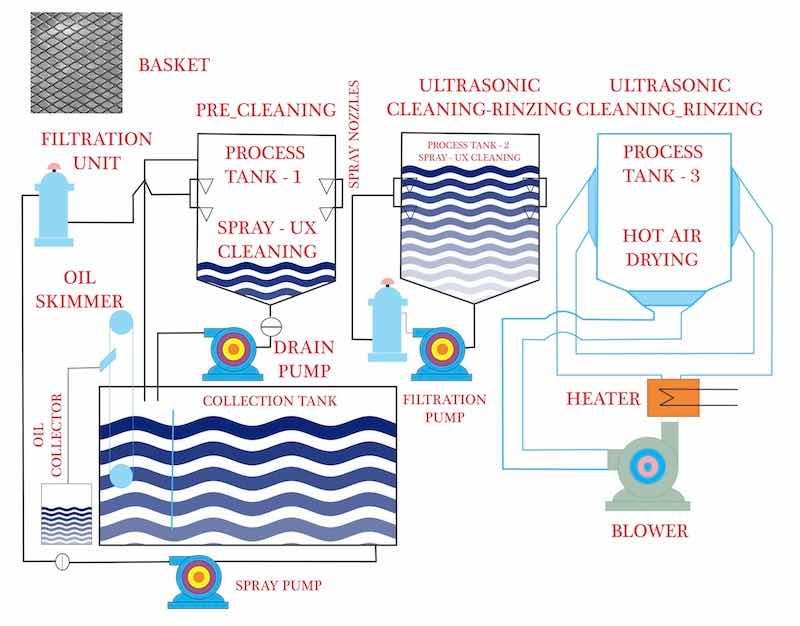 Image courtesy of Roop Ultrasonix Limited. https://www.rtulgroup.com
Image courtesy of Roop Ultrasonix Limited. https://www.rtulgroup.com
Ultrasonic Cleaning
This process takes the concept of soak cleaning, adding to it the use of high frequency sound or ultrasonic waves. A combination of chemistry and this form of energy will effectively remove a multitude of soils. There is a wide range of soils that should be identified. These are: oils, grease, buffing and polishing compounds, paraffins and waxes, abrasives, rouges, metallic chips, fragments and shavings. Ultrasonic cleaning especially lends itself to removing these soils that are mechanically embedded in recessed areas. Common critical cleaning sites of this type include lettering, designs and inner diameters of tubing,
At the heart of this technology is an ultrasonic generator. It produces ultrasonic energy, coupled with a transducer unit, changing the signal to mechanical energy. This in turn produces a favorable cavitation action, scrubbing parts clean. The transducers employed are normally 20 kHz, but for specialized applications, 40 to 100 kHz can also be used. In the working solution, the cavitation intensity increases as the temperature of the cleaning solution increases. This usually holds up to a solution temperature of 160°F (71°C). Beyond this temperature range cavitation steadily decreases.
It is important to rack or fixture parts with care. This will maximize exposure to sufficient levels of ultrasonic energy that in turn enhances the effectiveness of the cleaner chemistry. Consequently there is an optimal alignment of the transducer with respect to the parts. Routine maintenance of the transducer is important to maintain optimum performance. Design and fixturing of parts should promote good solution drainage, prevent air entrapment and allow for favorable penetration of ultrasonic energy.
Ultrasonic cleaning solutions usually provide formulations in the pH range of 8-13. Appropriate selection of the cleaner formulation is made to be compatible with the substrate to be cleaned. The cleaning of non-ferrous or more sensitive metals may also include inhibitors in the cleaner concentrate. Such metals that are commonly ultrasonically cleaned include: aluminum, brass, white metal and zinc. The ultrasonic cleaner may be the first step in surface preparation, or it may precede a soak cleaner. If simply cleaning the parts is the only requirement, then drying will be essential.
Mechanical dryers such as spin units, hot air knives or centrifugal types are commonly used. Most ultrasonic cleaning formulations impart a protective film over the basis metal. This film residue is visibly seen as water breaks. A soak cleaner dip is usually sufficient to remove this film, followed by proper drying. In a plating cycle, the soak cleaner may be followed by or substituted with an electrocleaner, to remove the inhibitor film. The parts being free rinsing with no water breaks, are clean and ready for surface activation before plating. It is also important that the correct electrocleaner is used, thereby avoiding tarnish, etching or pitting of the sensitive metal surface.
Mechanical Cleaning
This is a proven cleaning method, in use for rapid, effective removal of soils. It can be used for bulk, large volumes of parts. Cleaning is very effective in automated equipment. Painting, powder coating and phosphate lines make heavy use of mechanical cleaning. It is conducive to bulk cleaning of stamped and mechanical parts. Mechanical cleaning combines chemical and mechanical energy to remove soils.
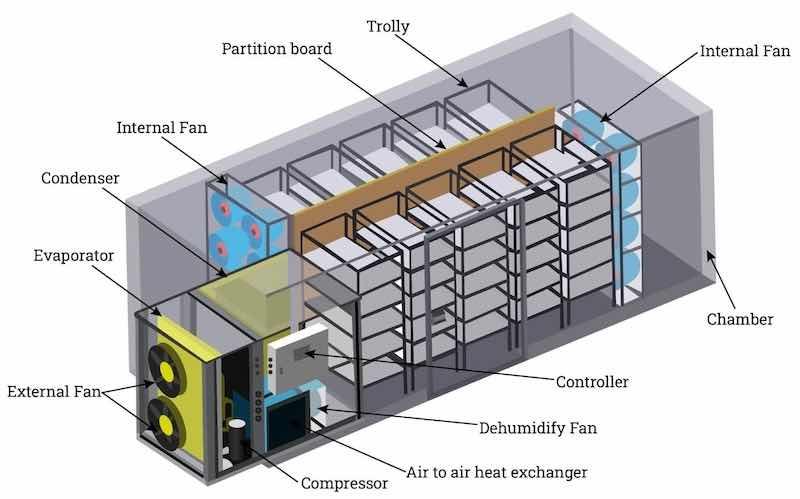 Image courtesy of www.IQSDirectory.com
Image courtesy of www.IQSDirectory.com
The lower temperature requirement of 120°F -150°F (49°C -66°C), reduces power requirements for heating. This is a big cost savings with respect to lowering energy demands. In addition, consumption of the mechanical cleaner may also provide additional cost savings. Spray and other appropriate forms of agitation during the cleaning cycle provide mechanical force, significantly improving the removal of soils. As such, the cleaner blends are low foaming, blended with powerful solvents (such as SARA Title III exempt types), dispersants and water conditioners (to prevent spray nozzle plugging). Based on the types of metals to be cleaned, the pH of a cleaner may range from near neutral to 14. Cleaner blends are available for all sensitive metals, both non-ferrous and ferrous types.
 New Holland Spin DryerThe desired mechanical action is critical to cleaning success. In spray cleaning, the pressure (PSI) of application is important for coverage of parts and improving the ability to dislodge soils. In wash machines, agitation trays or baskets of parts are placed in sealed compartments. Up / down, rotational, side-to-side action occurs. Racking of parts or placement in bulk tubs should be considered in parts configuration or design. This should incorporate accessibility to proper drainage of solutions and rinsing. Mechanical cleaners predominantly employ displacement of soils. Practical equipment advantages include recirculation tanks that can be continually skimmed to remove soils. Cost savings are realized by extending the cleaner bath service life and simplifying waste treatment.
New Holland Spin DryerThe desired mechanical action is critical to cleaning success. In spray cleaning, the pressure (PSI) of application is important for coverage of parts and improving the ability to dislodge soils. In wash machines, agitation trays or baskets of parts are placed in sealed compartments. Up / down, rotational, side-to-side action occurs. Racking of parts or placement in bulk tubs should be considered in parts configuration or design. This should incorporate accessibility to proper drainage of solutions and rinsing. Mechanical cleaners predominantly employ displacement of soils. Practical equipment advantages include recirculation tanks that can be continually skimmed to remove soils. Cost savings are realized by extending the cleaner bath service life and simplifying waste treatment.
Equipment Considerations
New mechanical cleaning lines or stations can be readily put in place. Design and space allocation can be determined as per most cleaning processes. Used equipment, in good operation, can provide welcome cost savings.
The overall capital expenditure can contribute to end-of-year business tax benefits. Typical criteria to consider include the basics of what is needed for the specific requirement, and converting to an aqueous cleaning operation. Heating can be electric immersion, insulating hot water, steam or gas-fired.
Dryers can be centrifugal or forced air. Mechanical washers may include single station spray cabinets, multi-station (three or five stage) and automated, rotating, sealed washers. Other considerations include energy sources to power equipment, work areas, plumbing, remodeling and expanding existing floor space.
Wet vs. Dry Parts
As described previously, a benefit of solvent cleaning is the discharge from the cleaning unit of clean and dry parts. When considering aqueous cleaning the cleaning time can be for example two to four times longer.
Having achieved clean parts, they are wet and may need drying, if only cleaning is the process cycle. This requires some form of drying equipment, such as hot air knives or ovens, as described in the previous section. The energy requirement for heat and equipment space allocation are essential along with the associated costs for installation and maintenance.
Stephen F. Rudy, CEF, is president of Chem Analytic and has written extensively about the finishing industry. Visit www.chemanalytic.com or call him at 917-604-5001.



































