Rust and corrosion inhibitors are characterized by their chemical type, optimum use, and related properties.
Types of corrosion inhibitors:
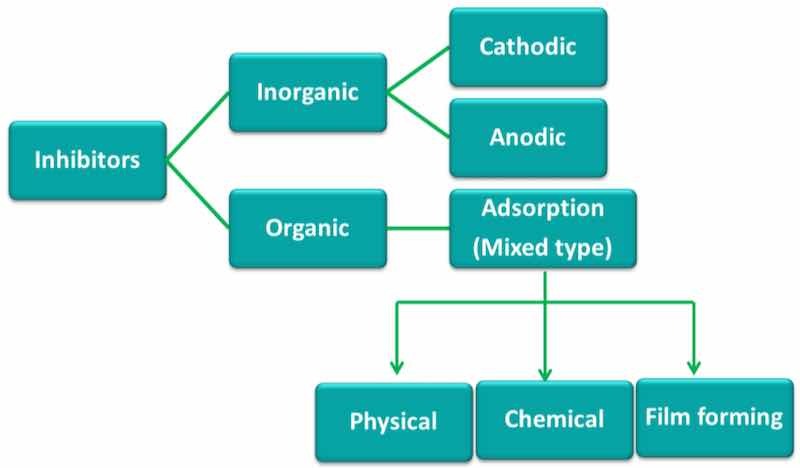
This class of products are geared specifically to retard or minimize corrosion of metals. The application is by coating bare metals, parts in transition towards a concluding finishing process, or as a final coating for sealing protection.
Targeted application for protection is based on the capability of each inhibition system to meet general or specific requirements whether indoors or outdoors. Storage atmospheric conditions or end use factors to consider for desired rust or corrosion protection include the condition of the basis metal (porosity, surface imperfections, etc.), activity, length of storage time, total finishing coatings, and relative humidity.
Examples of how they effectively protect metals:
- One class of corrosion inhibitors form a passivation layer, providing superior protection at a range of 30-200 angstrom thickness.The corrosive agents blocked include oxygen, hydrogen sulfide, and carbon dioxide.Benzotriazole is an example of a forming a superior passive coating on copper. It is referred to as a precipitation film type inhibitor. Long chain polymers, specifically of the fatty acid type are also of the film type inhibitors.
- Organic corrosion inhibitors retain a surfactant molecular structure.One portion of the molecule is termed hydrophilic, bonding to the conditioned metal.Another portion of the molecule is hydrophobic, blocking the diffusion of oxygen and water to the metal.
General information is given here for a group of rust inhibitors.
Water-Based
Concentrated formulations are available in powder and liquid form concentrates. They are generally of equivalent effectiveness based on the intended application. Most typically for steel, brass, and copper alloys, the working solutions can be applied by immersion, manual wiping, or by spray. There is no post-rinsing. Parts are permitted to dry as treated. The active rust, corrosion, or tarnish inhibitors coat the surface, are reactive to oxygen, thereby negating the potential oxidizing activity of oxygen in the air.
Parts can be racked, loaded in barrels or baskets, or hand processed. A common application is for temporary protection. Examples are in plant storage, short-term inventory, or before additional finishing or fabrication. When ready to proceed with additional surface treatments, cleaning in a warm or heated soak cleaner will normally remove the protective film. This is readily achieved in a plating, paint, or powder coat line.
If the parts are mass finished, as part of a process cycle, this may indeed impart surface protection. Most burnishing systems incorporate corrosion inhibitors, driving these protective films into the metal surface. Because of related environmental requirements and discharge limitations, compliant inner transition metals, specialty surfactants, and water shedders as formulated in commercial products, meet corrosion protection requirements. These effectively replace chromates, nitrites, and amines.
Water Displacement
Formulations based on this form of corrosion protection are solvent types containing inhibitors, oils, and surfactants. Concentrates are used at full strength or diluted with specific organic solvents. Evaporation and flash point considerations require that this group of rust inhibitors be used below 100°F (38°C). Cleaned parts are water-rinsed and then conditioned in the corrosion inhibitor.
Water is quickly displaced from the metal surface by a protective, transparent film. This film becomes a very effective moisture blocker by means of a mechanical barrier. Thickness and drying characteristics are unique and controlled for the degree or magnitude of surface protection required. The barrier or film may be dry-to-touch, slightly oily, or non-tacky. The condition of the film determines the degree of protection and related characteristics.
Dry-to-touch. Thickness may vary from under 0.1 to 0.2 mil. By completely drying on the metal surface, a transparent barrier is formed. Protection is approximately 50 hours per ASTM B117; one-month protection per ASTM D-1748.
Lightly oily. The film thickness approaches 0.2 mils. The film’s condition allows it to flow, covering or “healing” any metal surface exposed by mechanical action (scratching, abrading, etc.). Protection is close to 100 hours per ASTM B-117; one month of protection per ASTM D-1748.
Non-tacky. These are very thin, transparent protective coatings. The thickness ranges from 0.05 to 0.10 mil. Corrosion protection increases with increasing film thickness, up to 100 hours per ASTM B-117 to less than 20 hours; less than 20 days to approximately 30 days per ASTM D-1748.
Popular applications of these water-displacing dips follow black oxide and zinc phosphate processes. This provides Mil-spec-approved corrosion protection for thin oxide coatings. The color of the black oxide finish is accentuated. Zinc phosphate crystals absorb the oils.
Some oils are non-conductive where the application requires this property. The solvent content of the particular oil usually determines the drying time. Oiled parts, especially small ones, can be spin-dried.
Water Emulsifiable
Formulations based on this action are oils containing corrosion inhibitors and special emulsifying agents. The concentrate is mixed with water, forming a stable emulsion. The corrosion inhibition characteristics of the protective film vary with the oil-to-water ratio used to prepare the working solution.
Most of the water-emulsifiable systems include agents providing resistance to alkaline cleaner drag (as a contaminant). This prevents the breakdown of the emulsion, rendering the solution useless. These baths are used from 75 165°F (24 - 74°C). Increasing the temperature does accelerate the drying of parts. Dangerous solvent evaporation and ignition are avoided.
Waxy Emulsions
Waxy emulsions develop a clear, glossy, hard film on metal surfaces (finished or unfinished). The protective film provides some lubricity. Depending on the solution makeup, the film thickness varies from 0.1 to almost 0.5 mils. Concentrates can be used neat or diluted with water, forming stable emulsions. The surface condition can be accomplished by immersion, spray, or manual application. The bath temperature range is 70 - 85°F (21 - 29°C). Drying time is relatively short, facilitated by warm forced air or heated spin dryers. Being water-based, the wax emulsions have no flash point.
Table 1: Typical operating parameters and application
| Concentrate | Make-Up | Temperature | Time in min |
| Liquid | 1 - 10 vol% | 75°F - 80°F (24°C - 27°C) | 1- 5 |
| Powder | 2 - 6 oz/gal (15 - 45 g/L) | 75°F - 80°F (24°C - 27°C) | 1- 5 |
Table 2: Film and protection
| Oil in Water % | Film Characteristic | Salt Spray (ASTM B-117) |
| 5% | Dry-to-touch | 20 - 24 |
| 10% | Slightly oily | 40 - 50 |
| 20% | Oily | 90 - 125 |
With regard to environmental regulations, there is a continued driving force to reduce or eliminate VOCs as much as possible. New-generation oil-based rust and corrosion inhibitors feature compliance with related environmental regulations and mandates. These blends meet existing salt spray protection requirements as compared to the traditional oil-based systems. Proprietary formulations are in use and offer environmental along with process benefits.
Stephen F. Rudy, CEF, is president of Chem Analytic and has written extensively about the finishing industry. Visit www.chemanalytic.com or call him at 917-604-5001.



































