At times, words or phrases in surface finishing can generate loose terminology that may incorrectly define a specific activity or condition.
 Stephen Rudy CEFThis could relate to a descriptive or conditional situation. Sometimes, confusion may result, such as when the words qualitative and quantitative are used. Other examples may include bind versus bond, strip versus remove, empty versus purge, wet versus hydrate, and many other mixed terminology examples.
Stephen Rudy CEFThis could relate to a descriptive or conditional situation. Sometimes, confusion may result, such as when the words qualitative and quantitative are used. Other examples may include bind versus bond, strip versus remove, empty versus purge, wet versus hydrate, and many other mixed terminology examples.
In the finishing industry, we experience similar terminology twists. These can confuse meaning, intent, and how appropriate work or corrective action is applied. Let us consider some chemical descriptive terminology and its importance in waste treatment.
Chelating Agent
The chelating agent is typical of the organic acid type or pH-adjusted type, having more than one very active binding site. Some of the commonly referred to chelating agents used in metal finishing include EDTA (ethylenediaminetetraacetic acid sodium salt), NTA (nitrilotriacetic acid), citric acid, and cyanides (salts of sodium and potassium).
From a pure chemistry perspective, the chelating agent exhibits negative bonding sites, strongly attracting the positive metal ions, involving the inner orbital electrons. The resulting complexes are very strong and stable due to corresponding affinities for electronic stability.
For example, EDTA has been referred to as the world’s best metal ion chelator:
Metal ion (n+) + EDTA (4-) = MnY (n4-)
The above chemical equation shows the effect of strong binding of EDTA for the metal ion, resulting in a very stable chemical complex.
In layman’s terms, the chelating agent acts as a strong bear trap, tightly holding on to a soluble metal.
These chelating agents typically can be found in process groups, such as mass-finishing compounds, metal strippers, cleaners, plating salts, and additives. Their effects range from softening hard water to derusting and descaling, inhibiting chemical attacks on sensitive metals, complexing metals in cyanide-based plating solutions, and stabilizing metals in electroless plating.
Although essential to metal finishing processes, the presence of chelating agents can wreck waste treatment systems. The chelate-to-metal bond is very stable, even over a wide range of solution pH.
Over the years, effluent discharge limitations have become progressively tighter.
Very low parts per million (ppm) levels for most heavy metals are the norm. The addition can readily break these tight chelate-to-metal bonds of metal precipitants. Examples of these treatment agents are the sulfides and polysulfides. Their effect is to split the chelate-to-metal bond chemically in a specific pH range, forming the insoluble metal sulfide precipitate. This is the accepted procedure to convert soluble metals to their insoluble species. Metal precipitants are thus essential to optimize wastewater treatment.
Complexing Agents
These are inorganic, organic, and organic metallic compounds that bond to metal ions. However, they do so by having an affinity for outer electrons. This accounts for the somewhat weaker stability of the bond to metals compared to the previously discussed chelates.
Some complexing agents include tartaric acid, gluconates, functional amines, and certain phosphates. These chemical complexes are somewhat affected by solution pH and concentration. Applications in metal finishing include mass finishing, passivation of stainless steel, metal deposit stripping, surface preparation (cleaning and activation), aluminum treatment, and plating additives.
Their effects include hard surface cleaning, descaling and derusting, water softening, plating bath purification, inhibiting attacks on sensitive metals, and anti-corrosion action.
Complexed metals, if not treated properly, will also pose a big problem in wastewater treatment. However, as with metal chelate bonds, adding metal precipitants is very effective in breaking bonds in metal to complexing agents.
Specialty Treatments
In metal finishing operations, Hexavalent chromium and cyanide-containing waste waters are pretreated separately prior to introduction into the waste stream. Hexavalent chromium is reduced to its trivalent state by reaction at low pH (2.0-2.5) with sodium metabisulfite, a common additive for this reaction.
3NaHSO3 + 2H2Cro4 = Cr2(SO4)3 + 3NaHSO4 + 5H2O
This reaction is typically accomplished automatically by ORP
Cyanides are reacted with bleach (Sodium Hypochlorite 10-15%) in a two-step reaction by ORP to form a final carbon dioxide and nitrogen breakdown.
CN- + 2OH- = CNO- + Cl- + H2O (pH above 11)
2CNO- + 3OCl- + H2O = 2CO2 + N2 + 2OH- + 3Cl- (pH 8-8.5)
Complexing and Deflocculating Agents
These compounds may form complexes or suspend solid particles. Such particles can be rust, smuts, or precipitated metal hydroxides. The compounds include gluconic acid, glycolic acid, complex sugars, and colloidal materials such as glues and gelatins.
Complexes are formed at high pH (over 11) and higher concentrations, while deflocculation is favored at lower concentrations. Complexes and deflocculating agents slow the settling of precipitated solids in waste treatment. Organic polymers and inorganic salts are typically used to overcome this problem. This type of additive, combining both agents, is referred to as a coagulant.
Coagulation additives are already commonplace in a standard waste treatment system. These additives destabilize colloidal suspensions and neutralize charges, allowing metal hydroxide particles to begin settling.
As a final step in waste treatment, flocculants are added after the coagulation step. The flocculant is a polymer. Its effectiveness is in bridging the unstable colloidal particles. The particles will tend to settle quickly by forming agglomerated larger species, clarifying the treated water. Flocculants vary in molecular weight and charge densities for optimum application to specific wastewater treatment needs. Flocculants act to modify the space charge of particles, enhancing attraction to agglomerate particles into larger species to speed the settling of these large particles, as the illustration shows.
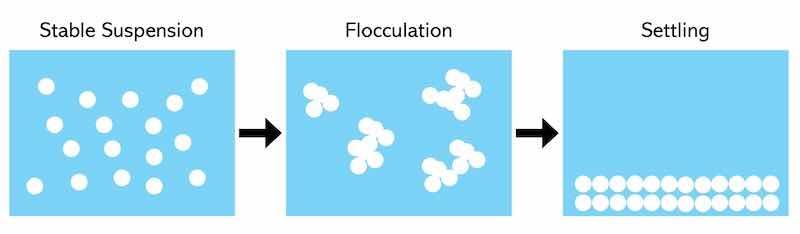
Treatment of spent solutions may also create foaming issues. The addition of defoamers can control this. These are typically silicon and silicon-free.
Jar testing is a typical evaluation used to determine appropriate chemistries and dosages before the waste treatment system is implemented. The example illustrates treatment from left to right. Completion is signified by the rapid settling of precipitated metal hydroxides and the clarity of water.
 Jar testing. Image courtesy of California State Water Resources Control Board.
Jar testing. Image courtesy of California State Water Resources Control Board.
Graph details optimum pH values for precipitation of metals as their hydroxides in wastewater treatment.
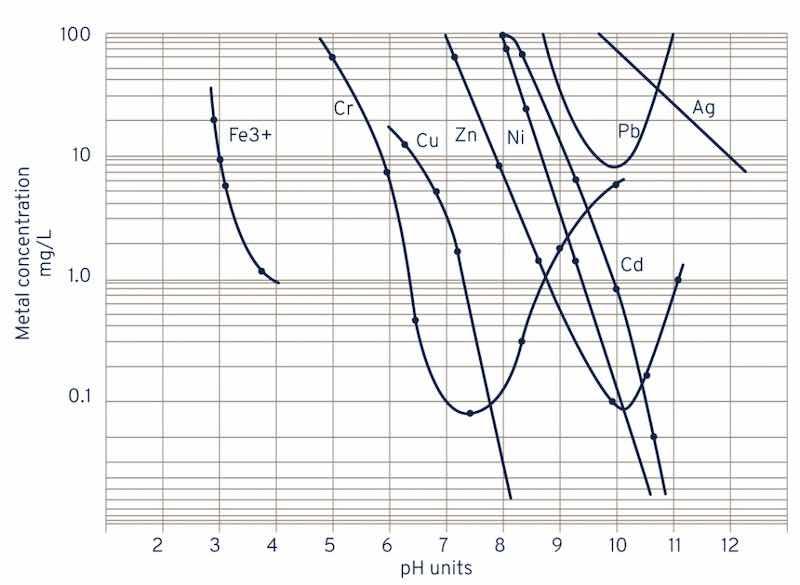 Image courtesy of Calix.
Image courtesy of Calix.
Years ago, chelates were first applied in electro-cleaning to descale steel. Complexes and de-flocculants replaced chelates to reduce formula costs and the demand for waste treatment.
Due to misused terminology, complexes, and de-flocculants were termed chelates when they were not. In this regard, it is important to understand that all chelating agents are complexes but that not all complexes are chelating agents.
Consequently, a cleaner may be referred to as a chelating type when, in effect, it may not be. Be certain of the products used by consulting your supplier and accompanying data sheets (SDS). It will clarify what is being used and how the specific chemistries may ease the burden on the critical wastewater treatment process.
Stephen F. Rudy, CEF, is president of Chem Analytic and has written extensively about the finishing industry. Visit www.chemanalytic.com or call him at 917-604-5001.





































